The total number of valence electrons in sulfur dioxide, SO2 2, is 18 electrons. This molecule is held together through covalent bonds. See full answer below. Become a member and unlock all.
CHEMISTRY SCHOOL- Oxygen and Sulfur atoms possess six electrons in their valence shells. Both of them come under the VIA group in the periodic table. By reading below you can check how we can calculate the total valence electrons in the molecule. Since, one molecule of sulfuric acid has 2 hydrogens (H) atoms, 1 sulfur (S) atom, and 4 of oxygen (0).
- Aug 04, 2019 In the case of Sulfur the valence electrons is 2,4,6. Now let's check the facts about Sulfur.
- The valence of sulfur in this compound has the value IV (+), because 4 electrons of the sulfur atom are shifted towards two oxygen atoms. The formula can be written as follows: S2O4, but by the rules it is necessary to reduce by 2. Dioxide when dissolved in water forms ions of weak sulfuric acid. Its salts - sulfites - are strong reducing agents.
Thiosulfate ion contains two sulfur atoms and three oxygen atoms. In lewis structure of S2O32- ion, there is -2 charge and oxygen atoms should hold them. Total valence electrons of sulfur and oxygen atoms are used to draw the structure.
Thiosulfate ion | S2O32-
Thiosulfate ion is one of the oxyanion of sulfur. Two sulfur atoms exist at two different oxidation states as +4 and +6. Also, overall charge of thiosulfate ion is -2. Therefore, there should be charges in atoms in thiosulfate ion.
Lewis structure of S2O32-
Steps of drawing lewis structure of S2O32-
Following steps are required to draw the S2O32- lewis structure and they are explained in detail in this tutorial.
- Find total number of electrons of the valance shells of sulfur and oxygen atoms
- Total electrons pairs
- Center atom selection
- Put lone pairs on atoms
- Check the stability and minimize charges on atoms by converting lone pairs to bonds.
Drawing correct lewis structure is important to draw resonance structures correctly
Total number of electrons of the valance shells of S2O32-
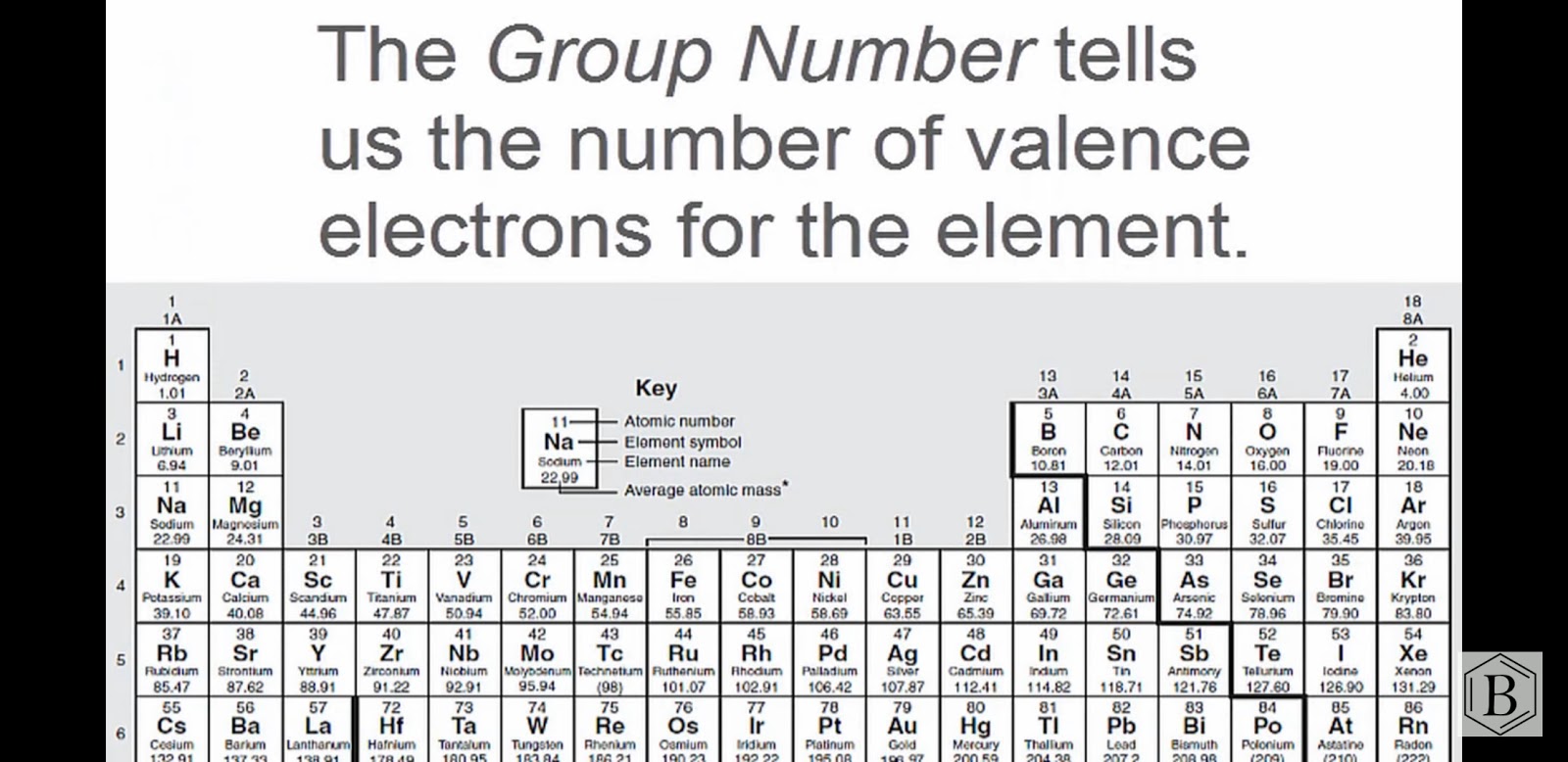
Both Sulfur and oxygen atoms are located at VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell.
- Total valence electrons given by two sulfur atoms = 6 * 2 = 12
There are three oxygen atoms in S2O32- ion, Therefore,
- Total valence electrons given by oxygen atoms = 6 *3 = 18
There are -2 charge on S2O32- ion. Therefore there are two more electrons which comes from outside to contribute to the total valence electrons.
- Total valence electrons = 6 + 24 + 2 = 32
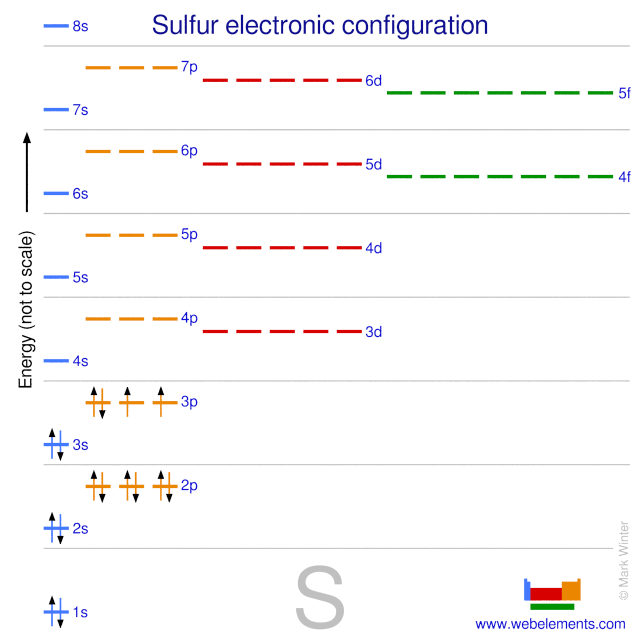
Sulfur Has 16 Valence Electrons

Total valence electrons pairs
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number total valence electrons by two. For, S2O32- ion, Total pairs of electrons are 16.
Center atom of S2O32- ion
To be the center atom, there are requirements.
- ability of having greater valance is important.
- Being the least electronegative element
Therefore sulfur has the more chance to be the center atom (See the figure) because sulfur can show valance of 6 and electronegativity of sulfur is less than oxygen. Maximum valence of oxygen is two. So, now we can build a sketch of S2O32- ion.
Around center sulfur atom, there is three oxygen atom and other sulfur atom.
Lone pairs on oxygen and sulfur atoms
- There are three S-O bonds and one S-S bond in the above sketch. Therefore only twelve (16-4 = 12) valence electrons pairs are remaining to complete the lewis structure.
- First, mark those thirteen valence electrons pairs as lone pairs on outside atoms (on oxygen atoms and sulfur atom). One oxygen atom and sulfur atom (located at outside) will take three lone pairs following the octal rule (oxygen atom cannot keep more than eight electrons in its valence shell).
- For three oxygen atoms and outside sulfur atom twelve electrons pairs are spent. Now all electron pairs (16) are spent. There is no electron pairs to mark on sulfur atom.
Charges on atoms
After, marking electron pairs on atoms, we should mark charges of each atom. Each oxygen atom will get a -1 charge and sulfur atom get a +2 charge. The overall charge of ion is ( -1*4 + (+2) ) = -2.
The Number Of Valence Electrons In Sulfur Is
Check the stability and minimize charges on atoms by converting lone pairs to bonds
When charges exist everywhere (on atoms) in the ion or molecule, that structure is not stable. We should try to reduce charges on atoms as much as possible. Now, we are going to learn how these facts will affect on sulfate ion.
- Oxygen atoms should hold negative charges because electronegativity of oxygen is higher than sulfur. Otherwise, we can say, ability of holding negative charges is great in oxygen atoms than sulfur atoms.
- The drawn structure is not a stable one because all atoms have charges.
- Now, we should try to minimize charges by converting lone pair or pairs to bonds. So convert one lone pair of outside sulfur atom to make a new S-S bond.
- Now there is a double bond between two sulfur atoms. Now, there are three S-O single bonds between sulfur atom and other three oxygen atoms.
You see charges of atoms are reduced in new structure. Now, there is no charge in outside sulfur atom and charge of center sulfur atom is reduced from +2 to +1. Charges on oxygen atom are remaining as same. However, charges of atoms are reduced. So we have an stable ion than out previous one.
Can I reduce charges of atoms furthermore?
You should know, sulfur can keep more than eight electrons in its last shell. Therefore we can convert one more lone pair of an oxygen atom to a bond to make a double bond between center sulfur atom and oxygen atom.
In new structure, charges of atoms are reduced than previous structure. Now there are no any charge on both sulfur atoms and one oxygen atom. Also, only two oxygen atoms have -1 negative charges. Now you understand this structure of S2O32- is more stable than previous two structures. So, this structure has more chance to be the lewis structure of S2O32- ion.
Lewis structure of S2O32- (thiosulfate) ion
Questions
Is number of lone pairs similar in S2O32-- and SO42- lewis structures?
Yes. Compare both lewis structures. Otherwise, we can think an oxygen atom of sulfate ion is replaced by a sulfur atom. Because both sulfur and oxygen belongs to group 6, their valence electrons in last shell is similar.
Can I draw the lewis structure of S2O32- putting a negative charge on sulfur atom?
Usually we dont put negative charges on electropositive atoms when there are strong electronegative atoms like oxygen. Oxygen's electronegativity is higher than sulfur. So we should put our negative charges on oxygen. Those structures are most stable ones.
what is the charge on oxygen atom in lewis structure of thiosulfate ion?
Two oxygen atoms have negative charges. Each od them have -1 charge and overall charge of thiosulfate ion is-2. There is no charge on remaining oxygen atom.
Number Of Valence Electrons In Sulfur Hexafluoride
structure of s2o32-
Lot of students struggle to draw lewis structure of S2O32- because there is two sulfur atoms. They cannot think, how sulfur atoms are joint with oxygen atoms in this molecule. There is no doubt to select sulfur as the center atom. So rest of oxygen atoms and sulfur atom should be around the center sulfur atom.
Number Of Valence Electrons In Sulfur Dibromide
Valence Shell Of Sulfur
Related Tutorials
Number Of Valence Electrons In Sulfuric Acid
SO32- lewis structure and resonance structures
NO3- lewis structure
NO3- resonance structures
NO2- lewis structure
N2O lewis structure, resonance structures
N2O5 resonance structures
Resonance structures examples
Nitrogen dioxide acidity