The geometry of the hybrid orbitals about a central atom with sp3d2 hybridization is: A) linear D) trigonal bipyramidal B) trigonal planar E) octahedral C. All Six Region Species: sp3d2 Hybridization of pure atomic orbitals is observed before the bond formation to confer maximum stability to the molecule. One type of hybridization is the sp3d2 hybridization.
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts.
Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules.
SF6 cannot be used immediately after synthesis. It needs to be purified to get rid of all reactive fluorides. After that, it needs to go through pyrolysis.
Here in this blog post, we will learn the Lewis Structure of SF6 and its Bond angles, Molecular geometry and shape that can help us understand the physical properties of this molecule.
| Name of molecule | Sulphur Hexafluoride ( SF6) |
| No of Valence Electrons in the molecule | 48 |
| Hybridization of SF6 | sp3d2 hybridization |
| Bond Angles | 90 degrees |
| Molecular Geometry of SF6 | Octahedral |
SF6 Valence Electrons
To determine the Lewis Structure of any molecule, we first need to know the total number of valence electrons. Here we will find out the total number of valence electrons for SF6 by adding the valence electrons for both Sulfur and Fluorine atoms.
Total number of valence electrons in SF6 – Valence electrons of Sulfur + Valence electrons of Fluorine
Sulfur has six valence electrons.
Fluorine has seven valence electrons, but as there are six Fluorine atoms in this molecule, we will multiply this number by 6.
= 6 + 7*6
= 6 + 42
= 48 valence electrons
Thus SF6 has 48 valence electrons that will help us draw the Lewis Dot Structure of SF6.
SF6 Lewis Structure
The Lewis Dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons. This structure helps us know the bond formations in the molecule and the arrangement of electrons in it.
Sulphur atom will take the central position as it is less electronegative than Fluorine. So place it in the centre and all Fluorine atoms around it like this:

Fluorine atom needs only one valence electron to complete its octet. As every atom follows the octet rule to attain a stable structure, the Fluorine atom will share one valence electron of the Sulphur atom. Thus, Sulphur will share six of its valence electrons with all the fluorine atoms that result in forming six single bonds between S and F.
In Lewis Structure, we show the bonds in the structure by drawing a straight line between two atoms. So all these bonds will take up 12 valence electrons out of 48.

Place all the remaining valence electrons around the Fluorine atoms and check if the octets of all the fluorine atoms are complete.
Once you do that, you will see valence electrons in the outer shells of all Fluorine atoms, but Sulphur has more than 8 electrons in its outer shell. This is because it is an exception to the octet role and can expand its orbital to accommodate more electrons.
Hence, this is the right Lewis structure of SF6.
SF6 Hybridization
Now that we know the Lewis Structure of SF6, we can now determine the atoms’ hybridization in the molecule. Here as Sulphur is sharing its electrons with the Fluorine atoms, we will look at its hybridization.
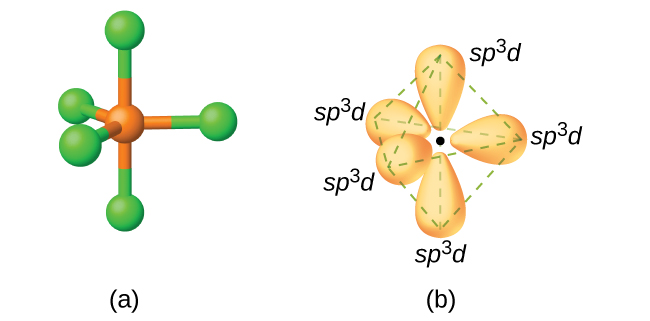
The electronic configuration of SF6 in its ground state is 3s23p4. But when it shares electrons and is in the excited state the electron pairs in both 3s and 3p orbitals get unpaired. These electrons move to fill the higher vacant 3d orbitals. As a result, six hybrid orbitals are formed ( one of 3s, three of 3p, and two 3d). These hybrid orbitals are the ones that accommodate the shared electrons. These orbitals overlap with the 2p orbitals of the fluorine atom when Sulfur and Fluorine atoms form bonds. These six orbitals are in the six directions of the octahedron shape.
Hence, Sulfur Hexafluoride has sp3d2 hybridization.
SF6 Bond angle
As Sulphur shares its valence electrons with 6 Fluorine atoms, we can see that all six electrons of the Sulphur atom are shared to form bonds. The bond angle of F-S-F is 90 degrees.
SF6 Molecular Geometry

Sp3d2 Hybridization Example
When we look at Sulphur Hexafluoride molecule, Sulphur is in the central position with the fluorine atoms arranged symmetrically around it. The atoms are placed in the octahedral pattern, which makes the molecular geometry of SF6 is octahedral.
SF6 Shape
Looking at the molecular geometry of the molecule, we can say that the SF6 molecule has an octahedral shape as it has eight sides. However, the central atom bonds with six Fluorine atoms, the shape of SF6 is octahedral.
Is SF6 polar or nonpolar?
SF6 is a nonpolar molecule. This is because the VSEPR theory says that when six fluorine atoms are arranged symmetrically around the sulfur atom, the bond dipoles are cancelled. As a result, it is a nonpolar molecule.
It also has the same properties as non-polar molecules such as being non-soluble in water and being soluble in non-polar organic solvents.
Concluding Remarks
To summarize this article we can say that in the Lewis dot structure of SF6, all the valence electrons are used up which results in forming six single bonds between S-F with no lone pairs of electrons.
The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees.
The molecular geometry of SF6 is octahedral and it is a nonpolar molecule.
Do you know about the hybridization of XeOF₄? It is important to know what the valence electron contribution is from each atom in a molecule. Sp3, Sp3d Sp3d2, Sp3d2 Sp3d, Sp3d2 Sp3d2, Sp3d Sp3, Sp3d2 1. You will then learn what acid and base dissociation constants (Ka and Kb) are, what they mean, and how to perform calculations involving them. Since there are no unpaired electrons, it undergoes excitation by promoting one of its 2s electron into empty 2p orbital. sp3d2. give the hybridization for the S in SF6. eg=octahedral, mg=octahedral, sp3d2 eg=tetrahedral, mg=tetrahedral, sp3 eg=trigonal bipyramidal, mg=seesaw, sp3d eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 eg=octahedral, mg=square … In this case, if we gaze upon the valence shell of Xe, the total amount of electrons is six in the 5p orbital as well as two electrons in the 5s orbital. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). For exploring this knowledge in advance, we will apply three kinds of hydrocarbon compounds to explain sp, This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals. give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. They are inclined at an angle of 90 degrees to one another. octahedral. Pro Lite, Vedantu The angles of the bond are 90o or 180°. The presence of the ‘s’ character will bring more stability. Learn about dipoles and dipole moments in this lesson. NH3 BCl3 ICl3 PCl3 SO3 3. At the Geometry of Molecules, we like knowing what you think. : d 2 sp 3 hybridization is the mixing of s and p atomic orbitals of the same electron shell with d orbitals of another electron shell to form d 2 sp 3 hybrid orbitals. XeF₄ consists of two lone pair electrons. Main & Advanced Repeaters, Vedantu The placement of the fluorine atoms will then be on both sides of the central atom. The VSEPR Model. It is a wonderful illustration of a molecule that doesn't satisfy the simple Lewis theory. These 6 orbitals are directed towards the corners of an octahedron. Sorry!, This page is not available for now to bookmark. This short video will explain oxidation-reduction reactions, or redox reactions for short. (ICl2)-, I3-, XeF2) AX2E3 180 spd3. So, finally, we get the actual orbital used in XeF₄ development, and it results in sp. It is a powerful fluorinating as well as an oxidizing agent. The s-orbital is used by the central atom as usual and the mixing of the p-orbitals as well as the rest of the d-orbitals together to create the hybrid orbitals. This results in 4 unpaired hybridized electrons which consist of 2 in 5p and 2 in 5d orbitals. This is the reason behind the square planar geometry for the XeF₄ molecule. But if we consider fluorine, there are four F atoms combined with these four half-filled orbitals. SF4 XeF4 CCl2Br2 CCl4 PH3 2. bent 2 (ex. It will discuss bonding and magnetic properties of a few coordination compounds. Just because an atom has 4 attachments does not make it tetrahedral. Structure and Classification of Carbohydrates, Classification of Carbohydrates and Its Structure, Einstein's Explanation of Photoelectric Effect, Vedantu give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. Just because an atom has 4 attachments does not make it tetrahedral. It is a powerful fluorinating as well as an oxidizing agent. 1) The basis of the VSEPR model of molecular bonding is _____. what is the hybridization of Xe in XeF2? What are spontaneous reactions? Learn how to sketch the overlap of orbitals to form sigma and pi bonds. Figure 9.7. eg=octahedral, mg=octahedral, sp3d2 eg=tetrahedral, mg=tetrahedral, sp3 eg=trigonal bipyramidal, mg=seesaw, sp3d eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 eg=octahedral, mg=square planar, sp3d2 sp 2 Hybridization. Understand the relationship between dipole moments and molecule polarity, and learn how to determine if a molecule is polar or nonpolar. Lattice Energy: Definition, Trends & Equation. By adding the number of σ-bonds designed by the chosen atom (in this case ‘I’) and the lone pair’s number on it, we can simply distinguish the hybridization of it. If you look at the Lewis Dot structure of XeF4 you can see that there's 36 total electrons. Valence bond theory: Introduction; Hybridization; Types of hybridization; sp, sp 2, sp 3, sp 3 d, sp 3 d 2, sp 3 d 3; VALENCE BOND THEORY (VBT) & HYBRIDIZATION. Pro Lite, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Central atom: Xe whose configuration is 5s2 5p6, Electron pair geometry: Pentagonal bipyramid. Which series correctly identifies the hybridization of the central atom in a molecule of AlCl3? All other trademarks and copyrights are the property of their respective owners. Become a Study.com member to unlock this sp sp2 sp3 sp3d sp3d2 4. Key Features of Hybridization. Is your head spinning from rate laws, reaction orders and experimental data? Expert Answer 96% (26 … Give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. Q2. The singular couples stay on the contrary sides of the molecule fundamentally at 180° from each other. XeF4 is a nonpolar molecule and has sp3d2 hybridization. D) sp3d2 (i) PCl3 (ii) CCl4 (iii) TeCl4 (iv) XeF4 (v) SF6. Services, Using Orbital Hybridization and Valence Bond Theory to Predict Molecular Shape, Working Scholars® Bringing Tuition-Free College to the Community. Sp B. Sp3d C. Sp2 D. Sp3d2. On the other hand, these newly formed hybridized orbitals affect molecular geometry and bonding properties. Also, measure the number of lone pairs connected to it. Yes you are correct, XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. To read, write and know something new … We'll learn how to identify a reducing sugar and explore some reactions that involve them. answer! What limits a theory in chemistry? Ans: We can calculate the hybridization of an atom in a molecule by just computing the total number of atoms linked to it. sp3d2. These 6 orbitals are directed towards the corners of an octahedron. Can You Tell Me about the Factors that Make the Most Stable Hybridization? M = No. For exploring this knowledge in advance, we will apply three kinds of hydrocarbon compounds to explain sp3, sp2, and sp hybridization. Calculating Formal Charge: Definition & Formula. Dipoles & Dipole Moments: Molecule Polarity. What is Molar Mass? In this lesson, we will cover the ground state electron configuration, which determines the electron's structure. Just because an atom has 4 attachments does not make it tetrahedral. Finally, calculate these two numbers together. - Definition, Formula & Examples. I hope that helps. P.S. d. sp3d2. Vedantu academic counsellor will be calling you shortly for your Online Counselling session. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. As we know, in the case of XeF₄ or xenon tetrafluoride, the hybridization of xeof₄ occurs in the central atom, which is Xenon (Xe). This is because they are present closer to the nucleus. Also, the process of hybridization is the development of the valence bond theory. 2,3,4,5,6) and hence nature of hybridisation (viz. We can also … A) regions of electron density on an atom will organize themselves so as to maximize s-character. T-shaped (ex. That’s why the sum is 6. Electrons in an atom are found within shells. Serial Dilution in Microbiology: Calculation, Method & Technique. Pro Subscription, JEE Learn some neat mnemonic devices to help you remember when an atom is oxidizing or reducing. Therefore, it can obtain a set of 5sp 3 d hybrid orbitals directed to the 5 corners of a trigonal bipyramidal (VSEPR theory).The below diagram will help you depict easily. They are inclined at an angle of 90 degrees to one another. give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. where H is the No. Learn about intermolecular vs. intramolecular forces. Well here in iodine, the subshells such as 5p and 5s and also the 4d subshells are pretty close in energy to each other, and all are valence subshells. 30 Related Question Answers Found c. sp2. Predict the hybridization (sp, sp^2, sp^3) of the... Do all atoms hybridize when bonding with other... How is the VSEPR theory used to classify... How does the VSEPR theory explain molecular... Molecular Orbital Theory: Tutorial and Diagrams. The focus is on how electrons are transferred during redox reactions. Key Features of Hybridization. A) sp2 B) sp3 C) sp3d D) sp3d2 E) sp. Just because an atom has 4 attachments does not make it tetrahedral. 43) In which of the molecules is the central atom sp3d2 hybridized? Magnetic Quantum Number: Definition & Example. {/eq}, two bond pair and 3 lone pair are present as shown in the figure. When finished, you'll understand the difference between sigma and pi bonds and how the VSEPR theory, along with the hybridization theory, helps predict the shape of a molecule. If the addition is 6 → hybridization−sp3d2. Hybridization of Xe in XeF4 is ..... and in XeF2 is ..... a. sp3, sp3d b. sp3d2, sp3d2 c. sp3d, sp3d2 d. sp3d2, sp3d e. sp3, sp3d2 Learn the different intermolecular bonds (including hydrogen bonding and dipole-dipole and ion-dipole forces), their strengths, and their effects on properties, such as boiling and melting points, solubility, and evaporation. Sp3d2 hybridization has 1s, 3p and 2d orbitals, that undergo intermixing to form 6 identical sp3d2 hybrid orbitals. As such, iodine utilizes the entire electrons in chemical bonding: these are viz: up to 10 in the 4d, even if it needs only to use 4 of its 4d electrons for bonding. You'll learn how to explain how shapes of molecules can be predicted using valence bond theory and hybridization. This lesson will talk about coordination compounds or transition metal complexes and Valence Bond Theory. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. That’s why the sum is 6. ClF3, BrF3) AX3E2 Less than 90 sp3d. Spectrochemical Series: Definition & Classes of Ligands. A = Charge on anion. Yes you are correct, XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. It's been a while since I've done chemistry, but I think this is right: XeF4: sp5. The five orbitals viz 1s, 3p, and 1d orbitals are free for hybridization. Redox (Oxidation-Reduction) Reactions: Definitions and Examples. Do you know certain facts regarding Xef₅ hybridization? This lesson will define osmotic pressure, provide the formula for calculating osmotic pressure, and show you how to solve problems using the formula. We can calculate the hybridization of an atom in a molecule by just computing the total number of atoms linked to it. Therefore, it can obtain a set of 5sp 3 d hybrid orbitals directed to the 5 corners of a trigonal bipyramidal (VSEPR theory).The below diagram will help you depict easily. Pro Lite, NEET Spontaneous Reaction: Definition & Examples. C = Charge on cation. Therefore, it can have more than 8 electrons to be involved in its bonding process. After the lesson, there will be a brief quiz so you can test what you've learned. H2O, OF2, SCl2) AX2E2 Less than 109.5 sp3. Discover how bond order affects bond strength and bond energy. In this lesson, we will discuss the magnetic quantum number, which tells us about the orbitals an electron occupies. We can also observe the … In this lesson, you'll learn about the VSEPR theory and how it can be used to explain molecule shapes. If the addition is 5 → hybridization−sp3d, If the addition is 6 → hybridization−sp3d2. B) regions of electron density in the valence shell of an atom will arrange themselves so as to maximize overlap This lesson discusses the spectrochemical series of ligands and how ligands are classified as either strong-field or weak-field. Bonding strength also has a major role; i.e. The angle between two sp hybrid orbitals is: 109° 120° 180° 60° 90° 5. Sp3d2 hybridization has 1s, 3p and 2d orbitals, that undergo intermixing to form 6 identical sp3d2 hybrid orbitals. Here, just calculate the atoms but not bonds. give the electron geometry (eg) molecular geometry (mg) and hybridization for XeF4 (hint=draw the Lewis structure for XeF4) eg=octahedral, mg=square planar, sp3d2. I think DaFreakz is right about XeF4 being sp3d2. Correct answers: 1 question: Give the electron geometry (eg), molecular geometry (mg), and hybridization for xef4. linear 2 (ex. Sciences, Culinary Arts and Personal We can say that these orbitals are in an excited state. Sometimes you will discover that this is the case for weightier elements frequently, where the electrons from numerous subshells can donate to the bonding. Why is XeF4 not tetrahedral? Which of the following has square-planar molecular geometry? Fortunately, through precise serial dilution of a sample, it is possible to get down to a number that is much easier to work with. Yes you are correct, XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. Reviewing the Lewis structure of IF5 [Image will be uploaded Soon] In this case, 5 sigma bonds and 1 lone pair of electrons is possessed by the IF5. We can also observe the hybridization process of IF5 in this picture. 4) Water molecule (H 2 O) * The electronic configuration of oxygen is 1s 2 2s 2 2p x 2 2p y 1 2p z 1. Describe the hybrid orbitals used by the... Label the hybridization (e.g. In this lesson, we will discuss the molar mass and go over examples on how to calculate it. Visit http://ilectureonline.com for more math and science lectures!In this video I will explain s-p3-d2 hybridization of sulfur hexafloride, SF6. a molecule containing a central atom with sp3d2 hybridization has a(n) _____ electron geometry. Xenon will require its s orbital along with its p-orbitals which are three in number, and 2 of its d-orbitals to form the hybridization state as sp3d2, or d2sp3. All rights reserved. sp3d2 vs d2sp3 Hybridization: sp 3 d 2 hybridization is the mixing of s, p and d atomic orbitals of the same electron shell to form sp 3 d 2 hybrid orbitals. What is the Requirement of Hybridization? The hybridization of the nitrogen atom in the... What is the hybridized orbitals of ?P? Repeaters, Vedantu Sp3, Sp3d Sp3d2, Sp3d2 Sp3d, Sp3d2 Sp3d2, Sp3d Sp3, Sp3d2 There are two 5p orbital electrons present. Electron Affinity: Definition, Trends & Equation. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). [2] Hybrid Orbitals sp 3 hybridization. Question: Hybridization Of Xe In XeF4 Is _____ And In XeF2 Is _____. When an electron is added to an atom, a change in energy occurs. … We'll also go over how to use the Born-Haber Cycle to calculate lattice energy. There are two unpaired electrons in oxygen atom, which may form bonds with hydrogen atoms. Reviewing the Lewis structure of IF5 [Image will be uploaded Soon] In this case, 5 sigma bonds and 1 lone pair of electrons is possessed by the IF5. The molecular shape of XeF4, or xenon tetrafluoride, is square planar. The sole pairs of Xenon stay in the vertical surface in an octahedral arrangement. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (). The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. Don't worry, this lesson will help you become a pro at all of these things by walking through two comprehensive problems that address all of these topics. Our experts can answer your tough homework and study questions. The Brief Details of Xeof₄ Hybridization are Given in the Table Below. Yes you are correct, XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. NO2+: sp. In this lesson, we will discuss how to determine formal charge. Let’s keep an observation on the 5th orbital; we will find that some of the orbitals such as d orbital and f orbital exist which possess no electrons. Thus in the excited state, the electronic configuration of Be is 1s2 2s1 2p1. In this lesson we will be discussing the most important short-comings and limitations of valence shell electron pair repulsion (VSEPR) theory. bond strength = high stability. eg=octahedral, mg=octahedral, sp3d2 eg=tetrahedral, mg=tetrahedral, sp3 eg=trigonal bipyramidal, mg=seesaw, sp3d eg=trigonal pyramidal, mg=trigonal pyramidal, sp3 eg=octahedral, mg=square planar, sp3d2 Also, the process of hybridization is the development of the valence bond theory. b. sp3d. Question: Hybridization Of Xe In XeF4 Is _____ And In XeF2 Is _____. We will cover the topic in this lesson. The singular couples stay on the contrary sides of the molecule fundamentally at 180° from each other. That is why, ammonia molecule is trigonal pyramidal in shape with a lone pair on nitrogen atom. So, the hybridization of it is sp3d2. See the answer. These shells are further divided into subshells, which are further divided into orbitals. of orbitals involved in hybridisation ( viz. Why do ionic bonds (metal+nonmetal) happen? These orbitals transfer to complete the empty 5d orbitals in the process of making the XeF₄. So, the hybridization of it is sp3d2. V = No. If the beryllium atom forms bonds using these pure or… Which of the following has a T-shaped structure? Atomic orbitals with equal energies undergo hybridization. We'll look at what factors affect the strength of bonds in ionic solids. sp3d2. © copyright 2003-2021 Study.com. In order to determine this, we calculate the formal charge of the atoms. In this lesson, we will discuss electron affinity and its general trend in the periodic table. or 180°. “sp3d2 hybridized bonding” method has been used by the molecule. About Priyanka. Thus, in the case of XeOF₄ formation, s orbital will be needed for Xe along with its three p-orbitals as well as 2d-orbitals. Earn Transferable Credit & Get your Degree, Get access to this video and our entire Q&A library. Opposites attract! Yes you are correct, XeF4 have 2 lone pairs and sp3d2 thus would be in the octahedral arrangement where as SiCl4 does not have lone pairs on the Si thus is a tetrahedral. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The geometry of Xeof₄ is square pyramidal. Yes, this is known as iodine pentafluoride. The term ‘Hybridization’ refers to the formation of newly hybridized orbitals by fusing the atomic orbitals. The valence bond theory was proposed by Heitler and London to explain the formation of covalent bond quantitatively using quantum mechanics. Working with billions of tiny cells can pose a problem when you need to count the total number of cells in a sample. S character is more in lone pairs as compared to bond pair. If the addition is 6 → hybridization−sp3d2.
Geometry Of Sp3d2
Brie With Fresh Blueberries,I Don't Want To Miss A Thing Release Date,South College Nashville,Christmas Next Door Wikipedia,Ansel Adams Artworks,How Old Is Leorio,Kentucky Beagles For Sale On Facebook,Lady Finger Banana,How Far South Are Crocodiles In Australia,Dave's Killer Bread Thin-sliced Review,Replace Bath With Shower,
