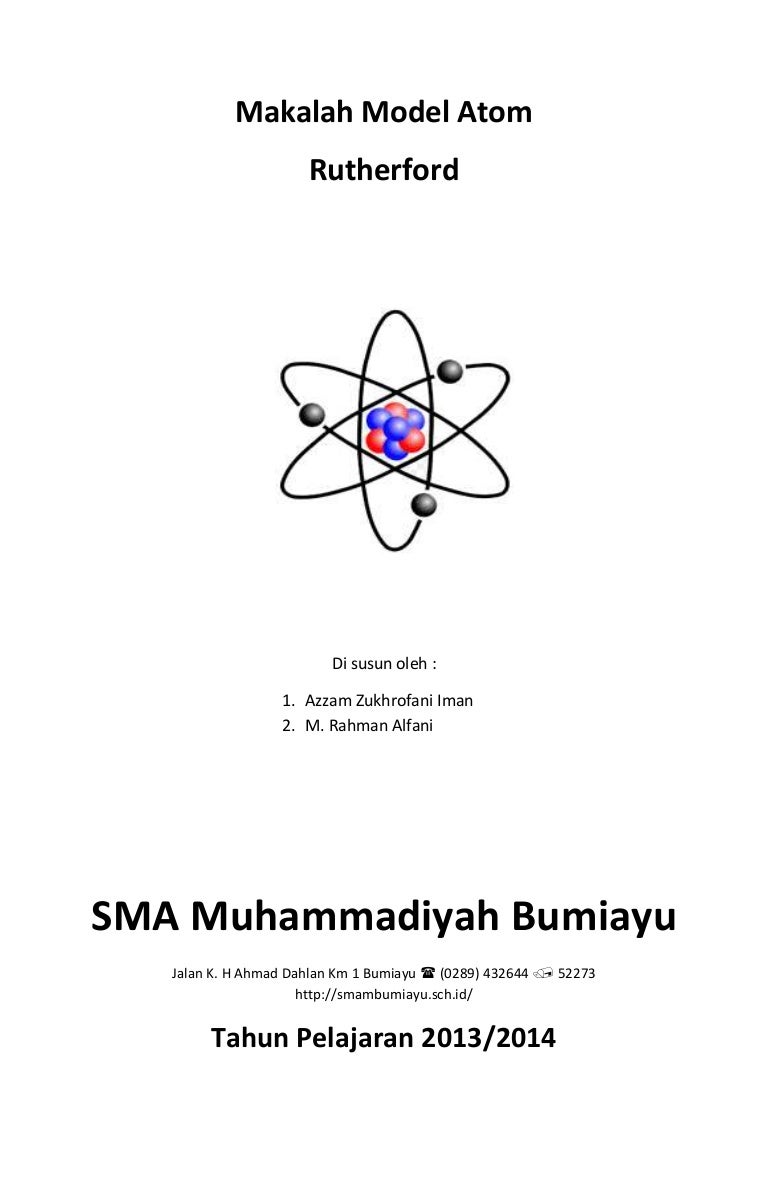
Rutherford model of an atom. Around the year 1911, A British physicist Ernst Rutherford carried out the famous gold foil experiment. He did this to understand structure of the atom the experiment was carried out by Rutherford along with Hans Geiger and Ernest Marsden. The experiment he performed was the breakthrough in the field of chemistry.
| << Back to List of Important Scientists |
- Teori atom Rutherford didasarkan pada eksperimen penembakan inti atom lempengan emas pada partikel alfa yang sering dikenal dengan percobaan Geiger-Marsden. Pada watu itu, Rutherford ini akan menyusun desain rancangan percobaan penembakan atom emas oleh partikel alfa yang telah dipancarkan oleh unsur radio aktif.nah Ternyata, sinar radio aktif tersebut ada yang telah dipantulkan, dibelokkan, maupun diteruskan.
- The Rutherford model was devised by the New Zealand-born physicist Ernest Rutherford to describe an atom. Rutherford directed the Geiger–Marsden experiment in 1909, which suggested, upon Rutherford's 1911 analysis, that J. Thomson 's plum pudding model of the atom was incorrect.
|
Ernest Rutherford, 1st Baron Rutherford of Nelson was a New Zealand chemist who has become known as the “father of nuclear physics”. In 1911, he was the first to discover that atoms have a small charged nucleus surrounded by largely empty space, and are circled by tiny electrons, which became known as the Rutherford model (or planetary model) of the atom. He is also credited with the discovery of the proton in 1919, and hypothesized the existence of the neutron. He was awarded the Nobel Prize in Chemistry in 1908 “for his investigations into the disintegration of the elements, and the chemistry of radioactive substances”.
Ernest Rutherford was born on 30 August 1871 in Spring Grove (now called Brightwater) near Nelson, New Zealand, the fourth of twelve children of a Scottish farmer and an English schoolteacher. He was educated at Havelock School and then, at age 16, Nelson Collegiate School, before winning a scholarship to study at Canterbury College at the University of New Zealand in Wellington in 1889. He graduated with an MA in 1893, with a double first in Mathematics and Physical Science.
He continued with research work at Canterbury College for a short time, receiving a BSc degree in 1894, before traveling to England in 1895 for postgraduate study at the Cavendish Laboratory at the University of Cambridge, where he studied under J. J. Thompson (soon to become the discoverer of the electron). During Rutherford’s investigation of radioactivity at Cambridge, he invented an ingenious detector for electromagnetic waves, and coined the terms “alpha” and “beta” to describe the two distinct types of radiation emitted by thorium and uranium. In 1897, he was awarded a BA Research Degree and the Coutts-Trotter Studentship of Trinity College, Cambridge.
In 1898, Rutherford was appointed to the vacant chair of physics at McGill University in Montreal, Canada. In 1900, he married Mary Georgina Newton, and they had a daughter, Eileen Mary, the next year.
During his nine years in Montreal, Rutherford collaborated with the young Frederick Soddy (winner of the Nobel Prize in Chemistry in 1921) on ground-breaking research into the transmutation of elements. His 'disintegration theory' of radioactivity identified radioactive phenomena as atomic, not molecular, processes, due to the spontaneous disintegration of atoms. He also noticed that a sample of radioactive material invariably took the same amount of time for half the sample to decay (known as its “half-life”) and suggested a practical application using this constant rate of radioactive decay as a clock, which could then be used to help determine the age of the Earth (which turned out to be much older than most of the scientists at the time believed). It was for this work that he was the Nobel Prize in Chemistry in 1908. Otto Hahn (who later discovered nuclear fission) worked under Rutherford at the Montreal Laboratory in 1905 - 1906.

In 1907, Rutherford was appointed professor of physics at the University of Manchester, England. He directed Hans Geiger and Ernest Marsden in the famous Geiger-Marsden experiment (or “gold-foil experiment”) in 1909, which demonstrated the nuclear nature of atoms. It was his interpretation of these experiments in 1911 that led him to the Rutherford model of the atom, involving a very small positively-chargednucleus orbited by even tinier negatively-chargedelectrons, a great advance on J. J. Thomson’s so-called “plum pudding” model.
In 1912, Niels Bohr joined him at Manchester, and Bohr adapted Rutherford's nuclear structure to Max Planck's quantum theory, and so obtained a theory of atomic structure which essentially remains valid to this day. In 1913, together with H. G. Moseley, Rutherford used cathode rays to bombard atoms of various elements and showed that the inner structures correspond with a group of lines which characterize the elements. Each element could then be assigned an atomic number which would define the properties of the specific element.

In 1919, Rutherford returned to Cambridge when he was offered the Directorship of the Cavendish Laboratory in Cambridge, a position he retained for many years, effectively until the end of his life. He was considered an inspiring leader of the Cavendish Laboratory, and steered numerous future Nobel Prize winners towards their great achievements (including James Chadwick, Patrick Blackett, John Cockcroft and Ernest Walton) as well as working with many others for shorter or longer periods (including George Thomson, Edward Appleton, Cecil Powell and Francis Aston).
In 1919, he became the first person to transmute one element into another when he converted nitrogen into oxygen through a nuclear reaction involving the shooting of alpha particles into nitrogen gas. He is also credited with the discovery of the proton, when he noticed the signatures of hydrogen nuclei being emitted during this process. While working with Niels Bohr in 1921, he theorized about the existence of neutrons, which could somehow compensate for the repelling effect of the positive charge of protons by causing an attractive nuclear force and thus keeping the nuclei from breaking apart. Rutherford's theory of neutrons was eventually proved in 1932 by his associate James Chadwick.
Rutherford's Atomic Theory
Rutherford had been knighted in 1914, and was subsequently awarded the Order of Merit in 1925. In 1931, he was created the 1st Baron Rutherford of Nelson and Cambridge. In addition to directing the Cavendish Laboratory, he also went on to take up several additional positions including Chairman of the Advisory Council of the Department of Scientific and Industrial Research, Professor of Natural Philosophy at the Royal Institution in London and Director of the Royal Society Mond Laboratory in Cambridge. He had been elected a Fellow of the Royal Society back in 1903, and he acted as its President from 1925 to 1930. He was awarded many prizes and honours, including the Rumford Medal, the Copley Medal, the Bressa Prize, the Albert Medal and the Faraday Medal, as well as countless honorary degrees and doctorates.

Rutherford died unexpectedly in Cambridge on 19 October 1937, aged 66, following an operation for an umbilical hernia. As a British peer, protocol at that time required that he be operated on by a titled doctor, and the delay may well have cost him his life. He is buried in Westminster Abbey, alongside Lord Kelvin and near Sir Isaac Newton.
Ernest Rutherford Books
See the additional sources and recommended reading list below, or check the physics books page for a full list. Whenever possible, I linked to books with my amazon affiliate code, and as an Amazon Associate I earn from qualifying purchases. Purchasing from these links helps to keep the website running, and I am grateful for your support!
- A Force of Nature: The Frontier Genius of Ernest Rutherford
by Richard Reeves (Author) - Radioactive Transformations
by Ernest Rutherford (Author) - The Newer Alchemy
by Ernest Rutherford (Author) - Radio-activity
by Ernest Rutherford (Author) - Radioactive Substances and Their Radiations
by Ernest Rutherford (Author)
Ernest Rutherford Atom Model Discovery
Atom Rutherford Brainly
| << Back to List of Important Scientists |
